Methods used in the Membrane Transport Physiology Lab
The Laboratory has established several key methodologies to study membrane transport processes pertinent to thyroid function.
|

|
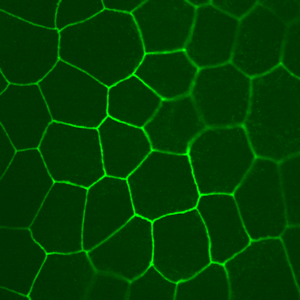
Thyroid follicles isolated from pig thyroids grow as high-resistance epithelial monolayers on permeable supports. Staining with an antibody directed against the tight junctional protein, ZO-1, is shown on the image to the left. To evaluate vectorial transport, the Laboratory performs electrical measurements of short-circuit current, as well as transepithelial voltage and resistance, using Ussing chambers (right). The Laboratory works with many different primary cell cultures in addition to pig thyroid cultures, as well as cell lines.
|
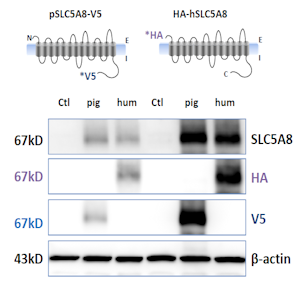
The Laboratory has established robust approaches for validating prospective antibodies to be used for protein biochemical applications. For example, to test cross-reactivity of an antibody generated against human SLC5A8 with pig SLC5A8, differentially tagged constructs of pig and human SLC5A8 were engineered and overexpressed in COS-7 cells. Immunoblot analysis with anti-tag antibodies, as well as the antibody in question, confirms cross-reactivity with porcine SLC5A8. The left and right sides of the blot represent two separate transfections with these constructs. |
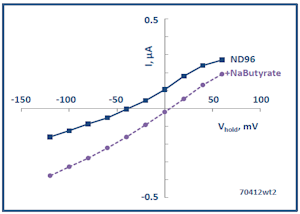
The Membrane Transport Physiology Laboratory also utilizes Xenopus oocytes for heterologous expression of transporter and channel proteins. Expression is analyzed using two-microelectrode voltage clamp. The panel at left shows a current-voltage plot showing the currents produced by expressed SLC5A8 in the presence and absence of butyrate, a substrate carried by this transporter (see below).
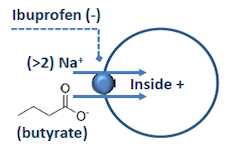
To study membrane protein interactions, the Laboratory also utilizes biochemical methods (immunoblotting, immunoprecipitation, surface biotinylation and confocal immunofluorescence microscopy).