One Health Newsletter
Strategies to Reduce Food Insecurity Associated with Global Antifungal Use
Authors
Noah Gold
Introduction
The emergence and spread of fungi in agriculture represents a growing social and public health problem. This multifaceted problem requires multidisciplinary solutions in line with the tests expected in our changing environments. In this context, I will highlight the risk to food safety that both fungal pathogens and high-risk treatment strategies pose.
Fungi and Agricultural Burden. Fungi are eukaryotic organisms broadly characterized by mold, yeast, and dimorphic forms. Regardless of replication strategy, many fungi externally release hydrolytic enzymes to digest available food sources.1 As common plant pathogens, fungi utilize plant hosts as a food source.2 Fungal pathogens are responsible for up to 20% of global crop loss and approximately $60 billion of lost revenue annually.3 This crop loss challenges food security for a rapidly growing population. Kettles and Luna (2019) predict that by 2044, humanity will require a “70% increase in global agricultural productivity” to feed over 9 billion individuals.3 However, they also report that increased crop production will increase the probability of plant pathogen emergence and limit food production.
Trends of Fungi Emergence. The burden of fungi will be compounded by rapidly rising temperatures and CO2 concentrations. Rising temperatures and CO2 concentrations are associated with increasingly virulent pathogens and decreased plant defense mechanism expression. Rising temperatures will also expand the habitable ranges of fungi and their burden on naïve plant populations. In combination with globalization, which has increased food demands and the probability of pathogen transport, climate change will “facilitate the emergence of new agricultural threats” by supporting fungi growth in previously impacted and new geographic regions.3 In addition to these abiotic factors, several farming strategies also facilitate the emergence of fungal pathogens and threaten food security.3,4
Modern farming practices are concentrated in specific geographic locations to grow genetically uniform monocultures and maximize product yield as well as economic profit.5 Concentrated monocultures are extremely prone to fungal epidemics and represent a critical vulnerability to food security. The predominant defense against these threats are antifungals.4
Antifungals: Therapeutic Strategy and Resistance. Fisher et al. (2018) report that 31 azoles represent the primary crop antifungal protection compounds and that many agricultural antifungals target the mitochondria, cytoskeleton, or ergosterol biosynthesis to eliminate fungal infections.4 However, the genomic plasticity and rapid reproduction rates of fungi facilitate the emergence of antifungal resistance in the absence of adequate stewardship.
Despite considerable antifungal use in agriculture, inadequate funding and research for novel compound discovery generated an “innovation gap” in treating diverse fungal infections.7 In combination with climate change and globalization, current antifungal practices increase the burden of antifungal resistance and necessitate investigation into diverse disease control strategies to fortify food security. 3,4
Methods
To investigate the influence of current antifungal strategies on antifungal resistance as well as global food security, this paper presents a holistic literature review and recommends methodologies to mitigate this threat. Sources used in this literature review include research articles, online resources, as well as personal communications with Georgetown University and United States Department of Agriculture employees.
Results
History of Antifungal Development. Antifungals have been used for over 150 years to control fungal infections in crops.4 Initially, antifungal compound identification required the screening of large molecules and associated product libraries to identify growth-inhibitive properties.6 However, the “low-hanging fruit” was rapidly discovered, and the development of antifungal compounds transitioned to synthetic tailoring.3 In synthetic tailoring, residual chemical groups of previously discovered compounds are altered to expand antifungal properties of the same core structure.7
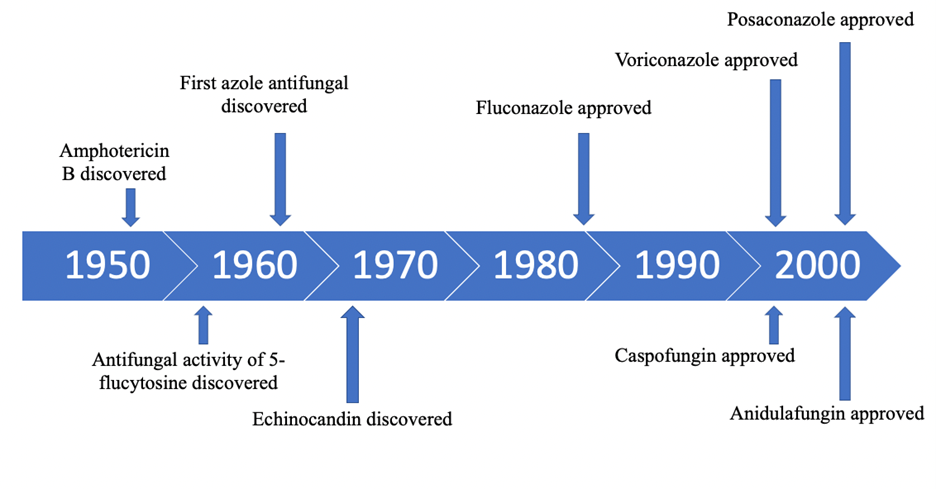
Challenges of Antifungal Development. The antimicrobial pipeline required to research and approve antifungal compounds disincentivizes novel drug discovery. Antimicrobial drug production requires extensive amounts of research, time, and funding to produce safe compounds. Due to this rigorous approval process, few compounds are deemed safe and marketable. Consequently, large markets must exist for these products to incentivize their development; however, these markets are limited by short-term product use. As a result, synthetic tailoring quickly outpaced alternative antifungal discovery due to these compounds being cheap, efficient, and rapid solutions to achieve disease control.7 Prioritization of synthetic tailoring and significant delays in novel antifungal approval generated an innovation gap in which large amounts of similar antifungal compounds were used to treat fungal infections (Fig. 1).6,7
Figure 1: Timeline depicting key milestones of antifungal drug development.Legend: The timeline highlights time gaps between antifungal discovery and antifungal drug production.
Source: Adapted from Roemer T & Krysan DJ (2014).6
Overuse of similar antifungal compounds in agriculture, during the interim of novel antifungal discovery, facilitated the rise of antimicrobial resistant pathogens (Fig. 2) that now threaten food security.4,8
Figure 2: Fungal species with reported antifungal resistance, by country.
Legend: Increasing color intensity reflects a growing number of reports. The plant maps depict spatiotemporal records of resistance of crop pathogens to azoles (blue scale). The human maps depict spatiotemporal records of resistance of the pathogens A. fumigatus, C. albicans, C. auris, C. glabrata, Cryptococcus gattii, and Cryptococcus neoformans to azoles (red scale).
Source: From Fisher et al. 2018.4 Reprinted with permission from American Association for the Advancement of Science.
Consequences of the Innovation Gap and Poor Antifungal Stewardship. Antifungal resistance is a heritable characteristic that develops due to selective pressures experienced by fungi. Poor stewardship of antifungals selects fungicide-resistant phenotypes that improve an organism’s ability to produce fit progeny in a given environment.4,8 Plastic fungal genomes and rapid reproduction rates promote population diversity and facilitate the emergence of antifungal resistance. Common mechanisms of antifungal resistance include mutation of the target protein, upregulation of the target protein or efflux proteins, and “detoxification by metabolic enzymes.” Fisher et al. (2018) report that “mutations resulting in conformational changes to the drug target site are the most common form of resistance in pathogenic fungi.”4,8 Considering these mechanisms, it is essential to investigate how current antifungal strategies have influenced the emergence of antifungal resistance.4,8
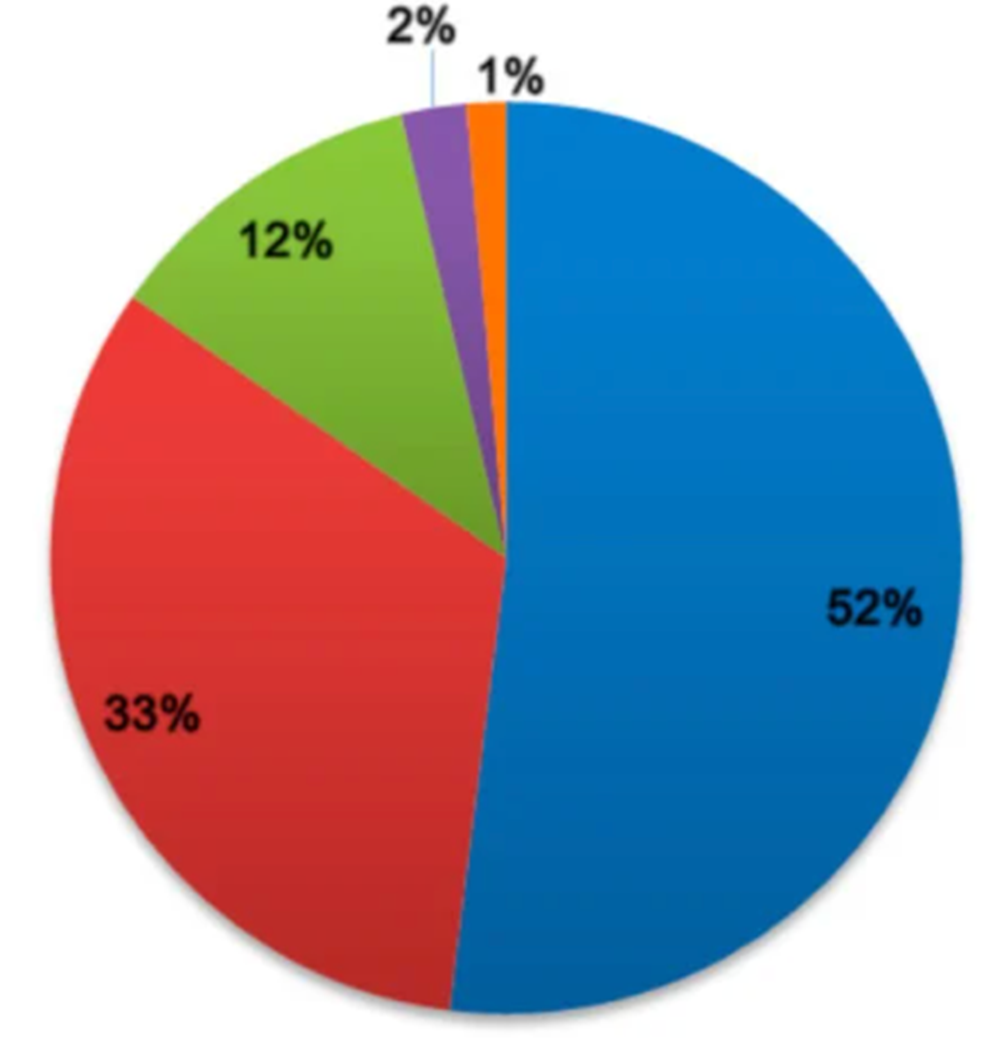
From 2012-2016, America represented 33% of global antifungal use in agriculture (Fig. 3).8
Figure 3. Use of pesticides in the world during 2012–2016 by continent.Legend: Blue=Asia; Red = America; Green = Europe; Purple = Africa; Orange = Oceania
Source: Licensee MDPI, Basel, Switzerland. This figure is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). Accessible to FAOSTAT. www.fao.org. 8
During this time, the U.S. utilized synthetic antifungal compounds such as azoles.4 The repetitive application of similar selective pressures increased the fitness of antifungal resistant fungi and facilitated the emergence of antifungal resistance. In the absence of novel antifungal research, the rate of fungicide resistance is outpacing the rate of antifungal discovery and development.4 In combination with globalization trends and increasingly prevalent abiotic factors that favor pathogen emergence, virulence, and host susceptibility, current antifungal practices threaten food security.3 Fortification of U.S. security against antifungal resistant pathogens can be accomplished by investing in novel antifungals, genomic technologies, and polyculture strategies. 3,4
Discussion
Poor antifungal stewardship is becoming “increasingly regarded as unsustainable," escalates antimicrobial resistance, and exacerbates the fungal burden on food security.3 Reflected by the European Union’s decision to eliminate the use of copper-based fungicides, antifungals alone cannot sufficiently control agricultural fungal disease.3 It is essential to invest in diverse disease control strategies that incorporate agriculturists’ willingness to adopt these tactics, accommodate the need to generate economic profit, and produce enough food to feed a rapidly growing population. To mitigate this vulnerability to food security, the following actions are recommended:3,4,7
- Investment into Genomic Technology as well as Novel Fungicide Research and Development. Diversified antifungal research and development can improve antifungal stewardship and reduce disease prevalence. As a familiar disease-control strategy, novel fungicides will likely be accepted by agriculturists and slow the emergence of antifungal resistance. In coordination with these efforts, improved genomic technology can target a new repertoire of antifungal compounds against exclusively susceptible pathogens. Kettles and Luna (2019) report that “omics technologies” represent diagnostic tools for pathogen identification.3 For these potential benefits, “novel agrochemicals” alone were graded to have a 5/10 impact score whereas improved “omics technologies” received an 8/10.3 Improved application specificity of novel antifungals represents an agreeable, short-term solution to remedy food security vulnerabilities.
- Develop Multi-Resistant Crops via Genomic Technology and Determine Long-Term Polyculture Profit Margins. Breeding disease-resistant plants is a common practice in agricultural communities. However, introducing multiple resistant genes into a plant, to grow multi-resistant crops, frequently reduces product yield and disincentivizes this practice.7 Consequently, the use of genomic technologies to incorporate multiple resistant genes into plants “without the yield penalty associated with conventional breeding” will likely be accepted by agriculturalists. Incorporation of multiple resistance genes into crops will decrease the prevalence of fungal infections and the need for antifungal application.3 These strategies received an 8/10 impact score suggesting that genomic technologies can adequately reduce the burden of antifungal resistance and improve food yield through a variety of mechanisms.3
Kettles and Luna (2019) also state that “mixed planting” reduces disease incidence and spread akin to the function of herd immunity.3 This long-term strategy received an impact score of 9/10 suggesting polycultures will effectively mitigate the threat of fungal infection and the need for antifungal use.3 However, the planting of less fruitful crops may sacrifice total food production for improved disease control. Potential loss of revenue may discourage farmers from polycultures. Given the need for a 70% increase in food production by 2044, a proposed study of “mixed planting” will investigate how the total food production of a polyculture compares to a monoculture when both are exposed to common fungal pathogens. The goal of this study will be to determine if polycultures can improve food production by protecting crops that would have otherwise been lost to fungal infection. If found to be profitable and improve overall food yield, farmers will be more likely to adopt these strategies that will mitigate antifungal resistance and improve food security.
Conclusion
In the absence of a “silver bullet,” the diversification of treatment options and prioritization of preventative strategies is essential to limit the burden of antifungal resistant infections and feed a growing population.3
Acknowledgment
This article was initially prepared for the Seminar for Global Infectious Diseases at Georgetown University. The author is grateful to his professors, Dr. Jean Paul Gonzalez for his guidance and support, and Dr. Richard Calderone for his inspiring work on fungal pathogens.
References
-
McGinnis MR, Tyring SK. Introduction to Mycology. In: Baron S, ed.Medical Microbiology. 4th ed. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
-
Dean R, Van Kan JA, Pretorius ZA, et al. The Top 10 fungal pathogens in molecular plant pathology [published correction appears in Mol Plant Pathol. 2012 Sep;13(7):804].Mol Plant Pathol. 2012;13(4):414-430. doi:10.1111/j.1364-3703.2011.00783.x
-
Kettles GJ, Luna E. Food security in 2044: How do we control the fungal threat?.Fungal Biol. 2019;123(8):558-564. doi:10.1016/j.funbio.2019.04.006
-
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security.Science. 2018;360(6390):739-742. doi:10.1126/science.aap7999.
-
Luster D. Lecture on U.S. Crops: Vulnerabilities, Threats, and Risks, Lecture presented at Georgetown University, January 31, 2023.
-
Roemer T, Krysan DJ. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014;4(5):a019703. doi:1101/cshperspect.a019703
-
Calderone R. Lectures on Antibiotic Pipeline & Food Security, Lectures presented at Georgetown University, October 4, 2022 & January 12, 2023
-
Brauer VS, Rezende CP, Pessoni AM, et al. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules. 2019;9(10):521. doi:3390/biom9100521
Next story: Breaking Silos: One Health in Action in the Philippines
One Health Newsletter
The One Health Newsletter is a collaborative effort by a diverse group of scientists and health professionals committed to promoting One Health. This newsletter was created to lend support to the One Health Initiative and is dedicated to enhancing the integration of animal, human, and environmental health for the benefit of all by demonstrating One Health in practice.
To submit comments or future article suggestions, please contact any of the editorial board members.