Pandemic: Learning From the Past, Be Prepared For the Future
Authors
Jean-Paul Gonzalez
Department of Microbiology & Immunology
Division of Biomedical Graduate Research Organization
School of Medicine, Georgetown University
Bien-Aime Mandja Makasa
Assistant Professor
University of Kinshasa
Megan Eppler
MPH, Infectious Disease/Zoonoses
GTA MPH program
Kansas State University (USA)
Mark Souris
Director of Research
French Research Institute for Development
This article will review the history of pandemics, their impact on medicine and public and veterinary health as well as on societies and politics when appropriate. This paper will show that only lessons of history can help humanity understand how environmental factors, information sharing, societal communities, human nature, and life sciences work together in tandem during pandemic conditions. These constitute the integral foundation needed for an explanatory approach of pandemics and the best way to prepare and respond to these scourges. This article will demonstrate sequentially how a transdisciplinary approach is perfectly adapted to the One Health concept. Therefore, it will follow the red line of pathogen emergence from the fundamental domains of a pandemic origin, sustained by some exemplary ones, and how we can be prepared for “The Coming Plague” (Garett, 1994).
Outbreak, Epidemic, Pandemic
First, let us define a few terms. An outbreak occurs in a community, while an epidemic appears to be limited to a country or to a health system of a territory (e.g., region, Province) with a risk of spreading beyond its borders to neighboring countries. The potential of disease extension lies principally on the increasing mobility and rapidity of transport of travelers and goods. The main difference between an epidemic and a pandemic is the geographic (territorial or continental) extent and incidence (referring to the population density) of both an infectious and a non-infectious disease.
Fundamentals of the Epidemic Risk
An epidemic (from the Latin “epidemic”, which means “at home”) corresponds to the result of an outbreak (sudden emergence of one or several clinical cases) with a rapid extension of a contagious disease among a large population. The epidemic is then limited to a region, a country, or a geographically well-defined area. On the other hand, a pandemic (Greek “pan” or “all” and, “demos” or “people”) is an extended epidemic that includes multiple outbreaks directly or indirectly, connected one to each other and threatening the entire population of one or more continents. A pandemic has specific risk factors of extension associated with trade and exchanges, population density (urban area), and access/availability of proper care from the health systems (disparity). The health consequences of a pandemic depend on the virulence and infectiousness of the pathogen, its impact on the health system, and the population vulnerability. The socio-political and economic consequences depend on morbidity and mortality among the workers of various economic sectors. The following section will illustrate the epidemic risks that have emerged in humanity and the lessons to be learned for the future.
From the Neolithic to the Industrial Revolution
Population density, mobility, and immunity are the three factors underlying the equation of epidemic risk, and ultimately, the threat of pandemic risk. Immune risk depends on the vulnerability (host susceptibility to infection), the chance of encounter between the host and pathogenic agent, and its characteristics (i.e., virulence, infectiousness). Altogether, this is what Sorre called a “pathogenic system” (Sorre, 1933) (Figure 1).
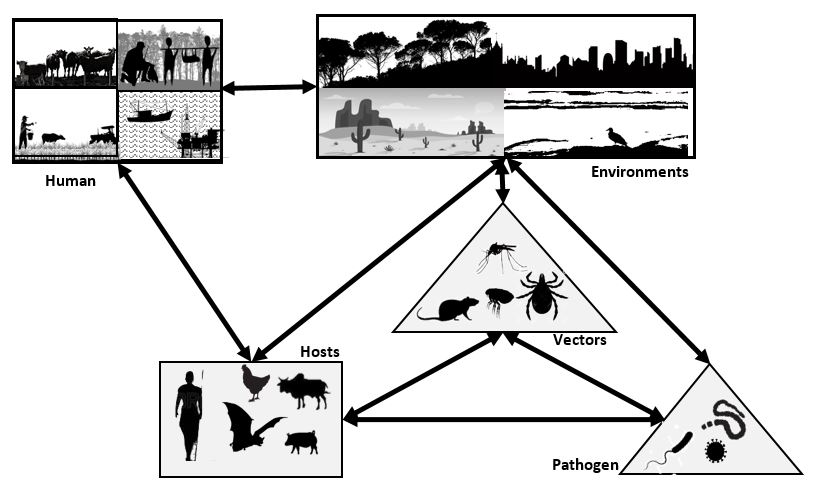
Figure 1. The Pathogenic Complex (Source: Jean-Paul Gonzalez, Bien-Aimé Mandja, Marc Souris, 2020).
The tree of evolution considers that bacteria and viruses existed before the appearance of man and probably before all other metazoan life forms on earth. Before the Neolithic revolution, at the start of the quaternary era, 1.5 million years before present (my BP) day (1.5 my BP), the first humans remained very dispersed and only on the Central Eastern African cradle (i.e. present day Omo Valley in Southern Ethiopia) of modern humanity. The first out-of-Africa migrations were possible thanks to cultural progress (fire control, subsistence rules, choice of game), allowing progression toward the Middle East 1.4 my BP (Kozlowski, 2005). A second great migration then reached South Asia to southern China around 0.7 my BP, and then, to Western Europe (0.6 my BP), toward Asia (0.4 my BP). All migrations consisted of isolated, nomadic tribes in which the pathogen could find its host, but whose structure (density) did not allow an outbreak - let alone an enlarged epidemic event. Due to this threshold of population density necessary for an epidemic manifestation, it would only appear when modern man began living in “cultural units” that an epidemic risk could occur (28,000 years BP). These cultural units also demonstrated a great deal of migratory power, but over long periods that were incompatible with pandemic risk. However, the Neolithic revolution (10,000 years BP) became a decisive moment that prepared man to gather and settle into an agricultural and pastoral lifestyle. Mobility around the settlements in search of/exchange of food and ceramics to preserve food ensured a constant diet. Constant nutrition greatly reduced mortality and promoted a remarkable increase in population density (Figure 2).
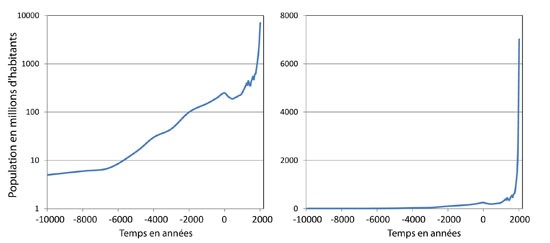
Figure 2. The Rise of Humanity: World Demography Evolution. A. Logarithmic Population Scale; B. Linear Population Scale. Population in millions of inhabitants as a function of time between 10,000 BCE and today. The values are estimates and the pre-1800 years BP (Before Present) values are to be taken as an indication. The curve shows a continuous increase of the world population with rapid growth during the 19th and 20th centuries that continues today. (Source: Cacharel & Harris, 1998)
The human population then increased significantly, close to 100 million individuals around 2,000 years BP. A maximum threshold of 250 million inhabitants was reached in the 1st century BP, then decreased to around 200 million at the end of antiquity. During the Middle Ages, the population began to increase again. By the 15th century, an estimated total global population of around 500 million people existed. Wars, famines, diseases, and epidemics (including plague, cholera, typhus) limited the growth of the Earth's population. Until about 1700, the number of humans increased slowly, struggling to reach 700 million. Nevertheless, the bar of a billion inhabitants on Earth was reached at the beginning of the 19th century (around 1825).
History and Pandemics
This section will review several important pandemics throughout the ages, further summarized in Table 1.
- Dating from 3,000 years BP, proof of a deadly epidemic ere find at the "Hamin Mangha" archaeological site in northeastern China, in which several houses were filled with skeletons. The cause of this apparent epidemic remains unknown.
- The Plague of Athens (430 years BP) took place at the start of a war between Athens and Sparta, lasted five years and resulted in 100,000 deaths. Researchers believe that the war overcrowded Athenian refugees that further exacerbated the epidemic.
- The Antonine Plague (165-180 Common Era, CE) lasted more than 15 years when the Roman soldiers came home after the war. The Antonine plague ravaged the army and killed more than five million people in the Roman Empire. This epidemic likely contributed to the end of the Pax Romana (Roman peace), a period which ranged from 27 to 180 CE. Instability then increased throughout the Roman Empire that lead to civil wars, "barbarian" invasions, and the rise of Christianity. This was a plague that significantly changed the course of human history.
- The Black Death (1346-1353 CE), caused by a highly pathogenic strain of the Yersinia pestis bacillus, crossed from Asia to Europe wiping away over half of the European population (Alchon, 2003; Flight, 2011; Panzac, 2010). This plague dramatically changed the course of European history due to the scarcity of a healthy workforce, prompting better wages and ending the serfdom system. The workers were better fed with improved quality products that contributed to innovation in the food chain across societal classes. Although pandemics succeed one another in history and seem to stand out in their time, they always carry the same stigma common to all societies. In one of these multiple allegories inspired by the epidemics of the past, Pieter Bruegel the Elder, in his immense painting of the "triumph of death" shows us how, where illness and death meet, society suffers in its entirety. Bruegel the Elder represents the economy with gold coins stolen by death, the people are represented by the king himself not yet dead, and the essential workers here are those in the funeral service, all affected and interconnected. We find all these actors in the lower left quadrant of the famous painting now located in the Prado Museum in Madrid (Figure 3).

Figure 3. “The Triumph of Death” 1562. Painting detail by Pieter Bruegel the Elder. (Source: Museo del Prado).
-
The cholera pandemics took a heavy toll on many generations, spreading around the world for the last 200 years. The first pandemic started in India in 1817 lasting until 1923. The next five cholera pandemics spread successively over time. After a transmission interruption of nearly 40 years, the seventh cholera pandemic occurred in 1961 (Figure 4). The pandemic slowly died down in the 1970s but continued to exist in modern middle- and low-income countries. Approximately 15 million deaths were recorded in India between 1817 and 1860 in the first three pandemics of the nineteenth century. Nearly 23 million people died between 1865 and 1917 during the next three pandemics (Hays, 2006; Svobodný, 2004; CBC News, 2010; Caeson & Waldman, 2019).
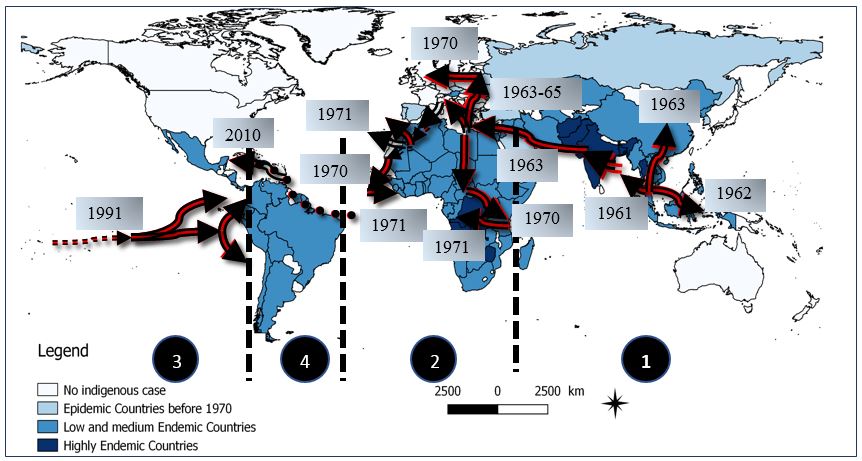
Figure 4. The seventh cholera pandemic: affected countries from 1961 to present. Arrows show the direction of spread of the epidemic, i.e. the population movements. From Indonesian Archipelago (1961), cholera spread to Western Pacific and South East Asia (1962-1965); 2. Cholera spread to Africa, Europe, Near and Middle East (1970-1971); 3. Cholera spread to Latin America (1991); 4. Cholera spread to Haiti and Dominican Republic (2010). (Source: Mandja, personal communication).
- The Spanish Flu (1918-1920) infected an estimated 40% of the world population (2 billion at the time) killing between 30 and 60 million people. The pandemic covered the entire planet causing several indigenous communities to be faced with extinction (Spreeuwenberg, 2018).
Recent Pandemic History (for this section, please see the Table 1 listed references)
- The acquired immunodeficiency syndrome (AIDS) pandemic (1981-present day) has taken the lives of around 35 million people since its discovery in 1981. The causative virus, human immunodeficiency virus (HIV), is likely zoonotic in origin and thought to have evolved from an African chimpanzee virus. Today, 40 million people are infected globally with more than half of that population living in sub-Saharan Africa. It took over 10 years to find an effective treatment and 40 years to record the first cases of permanent recovery (UNAIDS, 2020).
- Severe acute respiratory syndrome (SARS-CoV-1) became the first pandemic of the twenty-first century, affecting 26 countries and resulting in more than 8,000 cases and 774 deaths (Clark et al., 2018).
- The H1N1 swine flu pandemic unexpectedly emerged in Mexico (2009) and spread to the rest of the world, where 1.4 billion people were infected with an estimate of more than 500,000 deaths (Centers for Disease Control and Prevention (CDC), 2020).
- Ebolavirus virus (EBV) disease re-appeared in 2014 in West Africa and killed 11,325 people in Africa alone, while more than 28,600 cases were infected globally. Aside from coronavirus disease 2019 (COVID-19), other pandemics are still propagating since their emergence, like Zika virus disease in 2015 and chikungunya fever in 2013. Other diseases have been known for decades, still taking human lives globally. For example, poliomyelitis outbreaks are still active in Africa and Asia. A large outbreak of yellow fever is still circulating in Nigeria after emerging in 2017. Several countries are still reporting increasing numbers of dengue fever, such as Africa, Southeast Asia, the Middle East, Central and South America (CDC, 2019; Kindhauser et al., 2016).
Have we learned from past epidemics and those still present?
The saga of pandemics continues today and should keep us on the alert, never letting our guard down. Infectious diseases have swept the world for a very long time, killing millions of people, causing considerable historical upheaval, and transforming the future of entire populations. Scientists and communities have learned about the modes of disease transmission and pathogen virulence, using this basic knowledge to develop tools and strategies to combat epidemics.
In the last two centuries, microbiology has truly emerged as a science on its own with fundamental concepts created by Louis Pasteur, Robert Koch, and other numerous scientists. Several epidemics have been widely analyzed and yielded immense knowledge: the natural transmission cycle of yellow fever and its vaccine; dengue fever virus genotypes, their spread, and the host immune response; and the natural transmission cycle of EBV and how to respond and be prepared for outbreaks. Other pathogens are remarkable because of their vast epidemic expansion and increased risk of transmission beyond natural borders. Such diseases include hemorrhagic fevers, arboviruses (e.g., chikungunya fever, Rift Valley fever, West Nile encephalitis), and Zika-related diseases. All these emerging episodes seem very closely linked to the changing human and physical environments, resulting in explosive situations. Beyond the criteria directly related to the disease (virus, transmission, medical countermeasure available, etc.), it is the fear of those responsible for public health that have led to the desire of scientists to understand the threat and offer solutions to the public. Therefore, these often-unexpected epidemics pressure the health authorities to act as soon as possible in order to limit the damage the disease may cause.
Humanity must once again review the fundamental elements at the origin of new pathological emergences. As recently pointed out by Maurens and Fauci (2020), pandemics, such as COVID-19 are “not entirely new phenomena”, while several coronaviruses must have “emerged and spread pandemically in the era prior to the recognition of viruses as human pathogens”. Indeed, coronaviruses demonstrate the risk of interspecies jumping and acquiring a zoonotic profile (Figure 5). SARS-CoV-2 also surprised us with its extremely high infection rate (or its basic reproduction number, R0), a morbidity that overwhelmed the medical care systems of all affected states and countries, and the economic consequences never seen before.
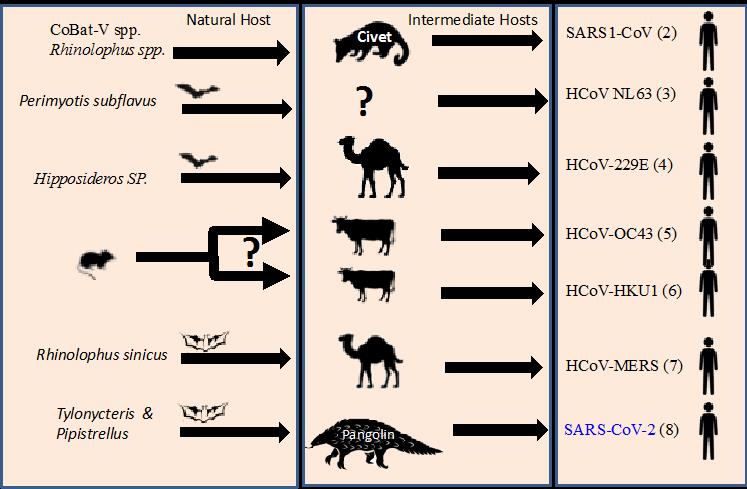
Figure 5. The Betacoronavirus infecting humans: SARS-CoV-1, MERS, SARS-CoV-2, and others: the putative role of an intermediate host.
Legend: (1) HKU2 = Rhinolophus bat coronavirus; Swine Acute Diarrhea Syndrome Coronavirus (SADS-CoV); (2) several bat species have been identified as a source of SARS1-Cov including: Rhinolophus sinicus, Rh. pearsoni, Rh. ferrumequinum, Rh. macrotis, Rh. pussilus; (3) Strong evidence for a common ancestor of the human virus to a coronavirus of North American tricolored bat (Perimyotis subflavus); (4) Hipposideros abae, H. ruber. (Corman et al. 2015); (5) common ancestor with bovine coronavirus (Vijgen et al. 2005); (6) Ye et al. 2020; (7) Goldstein et al. 2017; (8) Lau et al. 2020. (Source: Gonzalez, 2020, personal communication)
A One Health Solution
All these examples scattered throughout human history clearly show that the One Health concept is essential in understanding and responding to these epidemic emergencies, and in turn, the risk of pandemics. Indeed, we have seen how life sciences, social and human sectors, health decision-makers, economists and politicians have reacted (often in disparate ways) to the sudden, unexpected emergence of a pandemic. However, opinions vary on how to best react in order to mitigate the series of hardships generated by dramatic public health situations, disruption of supply chains, and the closure of borders.
Thus, within the framework of a “One Health” approach so that the affected society has the means to recover effectively when social and media resources are depleted and the existing health system shows weaknesses, the way forward will become obvious to decision-makers. The latter then, in health in the broadest sense, must seek scientific expertise to identify immediate solutions to current ills and to improve future “preparedness”. Research during this pandemic has been performed in a hurry and under pressure from all the societal sectors concerned. Therefore, a One Health policy must be put into perspective. Other epidemics are on the horizon. We must learn from the past in order to prepare ourselves for future emerging events, and above all, show strong resilience during these inter-epidemic periods when other priorities distract society and its decision-makers. Emergency funding is one of the main necessities during a pandemic response effort. Funds must be freed from administrative constraints and directed to the most local manifestation of the epidemic, as we learned from the recent EBV disease epidemics. It is necessary to anticipate international partnerships to manage the scale of these unpredicted disasters.
How ready are we for the next pandemic and prepared for the worst?
“...whenever a new, especially successful form of an infection emerges, it will spread rapidly around the globe.” - William H. McNeill, 1998, Plagues and Peoples
Diseases continue to emerge sporadically, particularly in Southeast Asia and South America, highlighting the imperfections in disease surveillance. Epidemics destabilize fragile governments, ravage the most vulnerable populations, and threaten the global community.
The ultimate goal of response is to develop a resilient global health infrastructure. The acquisition of treatments, vaccines/other preventive drugs, and biomonitoring remains essential to prevent the emergence and spread of any new disease. So far, only the states of the Western Hemisphere appear to have an extensive and well-established surveillance system. The World Health Organization (WHO), World Organization for Animal Health (OIE), and other international agencies favor global surveillance which confronts the disparity of states and peoples.
The world is in an intermediate phase of trying to reduce health disparities despite exponential population growth, political conflicts, migration, world trade, urbanization, and major environmental changes due to global warming. For the good of mankind, we must focus on building the capacity for health surveillance, for preparedness of epidemics, and for rapid, effective responses to any looming pandemics.
|
Disease |
Period |
Origin |
Expansion |
Death (MR)[i] |
|
|
Morbillivirus (Paramyxoviridae) (5) |
Measles |
Asia |
North Africa, India, China, Europe, and the Near East |
2m to 500,000 y. vii before XXth C vaccine (1 to 5%) [iv],[v] |
|
|
Salmonella enterica serovar Typhi (6) |
Plague of Athens / typhoid fever |
430-426C BCE |
Athens Peloponnese peninsula |
Eastern Mediterranean basin (Greece, Libya, Egypt, Ethiopia) |
75,000 to 100,000 y. |
|
Morbillivirus or Variola virus (7) |
Antonine Plague; Measle or smallpox |
165-180 CE3 |
Eastern Italia |
Asia Minor, Egypt, Greece, and Italia |
5 to 10 m for the period |
|
Morbillivirus or Variola virus (8) |
Plague of Cyprian Possibly measle or smallpox |
251-271 CE |
Rome |
Europe, Egypt (Luxor), Middle East |
>1 million y. (5.000/d. vii in Roma) |
|
Yersinia pestis (9) |
Plague of Justinian (Bubonic Plague) |
541-750 CE |
Egypt |
Europe, Byzantine Empire, Mediterranean Basin, worldwide |
25 to 100 m-/10,000 d. vii (40.0-50.0)[vi] |
|
Variola virus (Poxviridae) (10) |
Smallpox |
Xth C-1979[vii] |
North Eastern Africa |
Africa, Asia, Americas |
20m to 50 m y. |
|
Yersinia pestis (11) |
Black Death (Bubonic plague) |
1331(46)- 1353 |
Asia |
Europe, North Africa, and Asia |
75 to 200 m (10.0–60.0) |
|
Yellow Fevervirus (Flaviviridae) (12) |
Yellow fever |
XIVth C |
Africa |
Intertropical zone |
30–70m y. (3.0-7.7) |
|
Influenza A virus (Orthomyxoviridae) (13) |
First pandemic flu |
1510 |
Asia |
Asia, North Africa, Europe |
Unknown |
|
Variola major virus (14) |
American Plagues[viii] |
16thC |
Africa (trade) |
Central and South America |
(90% Western Hemisphere population) |
|
Yersinia pestis (15) |
Bubonic Plague |
1812-1819 |
Ottoman Empire (Asia) |
Occidental Asia, South-East and Central Europe, North and Horn of Africa |
>300,000 for the period |
|
Vibrio cholerae (Classic strain) (16) |
First Cholera pandemic |
1817-1824 |
Asia (India in Bengali) |
Asia, Europe |
>100,000 for the period |
|
Vibrio cholerae (Classic strain) (17) |
Second Cholera pandemic |
1826-1837 |
Asia |
Asia, Europe, North America |
>300,000 for the period |
|
Influenza A virus (Orthomyxoviridae) (18) |
Pandemic flu |
1847-1848 |
Asia |
Worldwide |
Unknown |
|
Yersinia pestis (19) |
3rd Pandemic Bubonic Plague |
1855-1960 |
China |
Asia, India, North America |
15 m and, >12 m India + China) |
|
Influenza A virus (Orthomyxoviridae) (20) |
Pandemic flu |
1857-1859 |
Europe |
Europe, North America, South America |
Unknown |
|
Vibrio Cholerae (Classic strain) (21) |
Third Cholera Pandemic |
1846-1860 |
India (Ganges River Delta) |
Asia, Europe, North America and Africa |
1m; 23,000 UK for the period |
|
Vibrio Cholerae (Classic strain) (16) |
Fourth Cholera Pandemic |
1863-1875 |
Asia |
Europe, North America, Africa |
600,000 for the period |
|
Variola major (22) |
Smallpox pandemic |
1877-1977 |
Worldwide |
Worldwide |
500 m for the period |
|
Vibrio Cholerae ( Classic strain) (16) |
Fifth Cholera pandemic |
1881-1896 |
Asia |
Asia, Africa, Europe, South America |
298, 600 for the period |
|
Influenza A virus subtype H3N8 (Orthomyxoviridae) (23) |
Russian Flu or Asiatic Flu |
1889-1890 |
Uzbekistan (Bukhara) |
Bukhara in Central Asia (Turkestan), Athabasca in northwestern Canada, and Greenland |
1m + for the period |
|
Vibrio Cholerae (Classic strain) (24) |
Sixth Cholera pandemic |
1899-1923 |
Asia |
Europe, Asia, Africa |
800,000+ for the period |
|
Poliovirus (Enteroviridae) (25) |
Poliomyelitis |
1900-1955 |
Western hemisphere |
Worldwide |
600.000 /y. (5-15) |
|
Encephalitis lethargica (26) |
Encephalitis lethargica pandemic |
1915-1926 |
Asia |
Worldwide |
1.5 million for the period |
|
H1N1 Influenza A (Orthomyxoviridae) (27) |
Spanish flu |
1918-1919 |
US Kansas (Fort Riley) |
Worldwide |
20 to 100m for the period (1-3)[ix] |
|
H2N2 Influenza A (Orthomyxoviridae) (28) |
Asian flu |
1956-1958 |
China (Guizhou) |
Singapore, Hong Kong, United States. |
1.1m for the period |
|
Vibrio Cholerae (El Tor strain) (16) |
Seventh Cholera pandemic |
1961–1975 |
Asia (Indonesia) |
Worldwide |
Unknown |
|
Marburg virus, Filovirus (Filoviridae) (29) |
Marburg disease |
1967[x] |
Africa, Europe |
3 (endemic) + 7 countries (imported cases) |
>500 for the period (88) |
|
H3N2 Influenza A virus (Orthomyxoviridae) (28) |
Hong Kong flu |
July 13 1968-1969 |
Hong Kong |
Singapore, Vietnam, Philippines, India, Australia, Europe, United States |
1 - 4m for the period (0.03) |
|
CCHF Virus, Nairovirus (Nairoviridae) (30) |
Crimean Congo Hemorrhagic Fever (CCHF) |
1969[xi] |
Central Africa |
Asia, Africa, Middle East |
>1,000 y. (5.0 to 4.0) |
|
Lassa virus, Arenavirus (Mammarenaviridae) (31) |
Lassa fever |
1969[xii] |
West Africa (Nigeria) |
- 7 endemics + 9 exported |
5.000 for the period (15 to 50) |
|
HIV-1, −2 (Lentiviridae) (32) |
AIDS pandemic |
1980s-present |
Cameroon |
Worldwide |
32m for the period (88)[xiii] |
|
Phlebovirus (Bunyaviridae) (33) |
Rift Valley fever |
1987-2012 |
Northeast Africa |
Africa, Western Asia[xiv] |
>260 for the period (50.0) |
|
SARS-Cov-1 Coronavirus (Coronaviridae) (34) |
SARS |
2002-2004 |
Guangdong province, China |
Worldwide (China, Vietnam endemic - 29 country exported cases) |
774 for the period (11.0) |
|
H1N1 Influenza A virus (35) |
Swine Flu pandemic |
2009-2010 |
Mexico |
North and Central America, Europe, Asia, Japan |
151,700 to 575,400 for the period (0.001 to 0.007) IX |
|
MERS-Cov Coronavirus (Coronaviridae) (36) |
Middle East respiratory syndrome |
2012-present |
Saudi Arabia |
Africa (Nigeria, Ethiopia, Tunisia, Canary Island), Arabian Peninsula, Oman, Qatar, Jordan |
300-500 for the period (37,10) |
|
Ebolavirus, Filovirus (Filoviridae) (37) |
Ebolavirus Disease |
2014-2016 |
Guinea (West Africa) |
[xv]Liberia, Sierra Leone Imported: Nigeria, Mali, Senegal, United States, Europe |
11,325 for the period (25.0 to 90.0) |
|
Zika virus (Filoviridae) (38) |
Zika Virus epidemic |
2015-present |
Africa/Indian Ocean |
Worldwide (Inter tropical zone) |
>500,000 for the period (0.02-0.09) |
|
SARS-Cov-2, Coronavirus (Coronaviridae) (39) |
COVID-19 |
2019-present |
China (Wuhan) |
Worldwide (210 countries reached as of 30 May 2020) |
375,000 for the period [xvi] (3.00) |
Table 1. Pandemics through the centuries (Please see the references (#) of Table 1 listed at the end of this article).Table 1. Legend: [i] Total or estimated number of cases (Case Fatality Rate percentage);[ii] C. = Century
[iii] BCE = Before Common Era; CE = Common Era
[iv] Uncertainty about virus circulation and endemics
[v] m = million
[vi] Of European population
[vii] y = year / d = day
[viii] Cluster of Eurasian diseases brought to the Americas by European explorers
[ix] Of the World population
[x] Germany, Yugoslavia and then discovered in Africa
[xi] Occurred South of 50 °N latitude then extended to the Middle East, Balkans, Asia
[xii] Imported cases to Canada, Germany, Israel, Japan, Netherlands, United Kingdom, USA
[xiii] Before Anti-Retroviral Therapy, ART
[xiv] Expansion to West Africa and Middle East and Western Asia (Saudi Arabia, Yemen)
[xv] Originally continental sparse repetitive epidemics in different countries of Central Africa, expansion within the African Rain forest
[xvi] as of June 1st, 2020
References
-
Garrett, L. (1994). The coming plague: newly emerging diseases in a world out of balance.
-
Sorre, M. (1933). [Pathogen complex and medical geography] In french Complexes pathogènes et géographie médicale. Annales de Géographie, 42, 235, 1 –18. French.
-
Kozlowski, J.K. (2005). [The first human migrations and the first stages of the settlement of Europe]. Diogène, 3(211):9-25. French.
-
Cachel, S. Harris, J.W. Petraglia, M.D. & Korisettar, R. (1998). The lifeways of Homo erectus inferred from archaeology and evolutionary ecology: a perspective from East Africa. Chapter 4 in Early human behaviour in global context: the rise and diversity of the lower. Korisettar R. & Petraglia M.D. Edit. Paleolithic record, 108 –132. DOI : https://doi.org/10.4324/9780203203279
-
Düx, A., Lequime, S., Patrono, L.V., Vrancken, B., Boral, S., Gogarten, J.F., Hilbig, A., Horst, D., Merkel, K., Prepoint, B., Santibanez, S., Schlotterbeck, J., Suchard, M.A., Ulrich, M., Widulin, N., Mankertz, A., Leendertz, F.H., Harper, K., Schnalke, T., & Calvignac-Spencer, S. (2019). The history of measles: From a 1912 genome to an antique origin. BioRxiv, 2019.12.29.889667.
-
Biello, D. (2006, January 25). Ancient Athenian plague proves to be typhoid. Retrieved August 15, 2020 from https://www.scientificamerican.com/article/ancient-athenian-plague-p/
-
Murphy, V. (2005, November 07). Health | Past pandemics that ravaged Europe. Retrieved August 10, 2020, from http://news.bbc.co.u/2/hi/health/4381924.stm
-
Harper, K. (2017, November 01). Solving the mystery of an ancient Roman plague. Retrieved June 18, 2020, from https://www.theatlantic.com/science/archive/2017/11/solving-the-mystery-of-an-ancient-roman-plague/543528/.
-
Little, L.K. (2007). Plague and the end of antiquity: the pandemic of 541-750. Cambridge: Cambridge University Press in association with the American Academy in Rome.
-
De Cock, K.M. (2001). The eradication of smallpox: Edward Jenner and the first and only eradication of a human infectious disease. Nature Medicine, 7(1), 15 –16.
-
Alchon, S.A. (2003). A pest in the land: new world epidemics in a global perspective. University of New Mexico Press. ISBN 0826328717
-
Chisholm, H. (1911). Yellow fever. In Encyclopedia Britannica (11th ed., pp. 910-911). Cambridge University Press.
-
Morens, D.M., North, M., & Taubenberger, J.K. (2011). Eyewitness accounts of the 1510 influenza pandemic in Europe. The Lancet, 376(9756), 1894-1895.
-
Flight, C. (2011, February 17). History - British History in depth: Smallpox: Eradicating the scourge. Retrieved June 20, 2020, from http://www.bbc.co.uk/history/british/empire_seapower/smallpox_01.shtml
-
Panzac, D. (2010). Plague. In Gábor Ágoston and Bruce Maters Edit., Encyclopedia of the Ottoman Empire (pp. 463). Infobase Publishing. ISBN-13 : 978-0816062591
-
Hays, J.N. (2006). Epidemics and pandemics: Their impacts on human history. Santa Barbara, CA: ABC-CLIO.
-
Svobodný, P. (2004). The health of the population and health policy in 19th century Bohemia: The case of Asiatic cholera (1830s–1900s). European Health and Social Welfare Policies, 200−215
-
Thomas, P.B. (1812-1882). On the influenza, or epidemic catarrhal fever of 1847-8. [electronic resource: https://archive.org/details/b21307866] St. Thomas's Hospital. Medical School Library former owner; King's College London.
-
Stenseth, N.C. (2008). Plague through history. Science, 321(5890), 773-774.
-
Beveridge, W.I. (1978). Influenza, the last great plague. London: Heinemann.
-
CBC News. (2010, October 22). Cholera’s seven pandemics. Retrieved August 2020, from https://www.cbc.ca/news/technology/cholera-s-seven-pandemics-1.758504
-
Henderson, D.A. (1975). Smallpox eradication—the final battle. Journal of Clinical Pathology, 28(11), 843.
-
Center for Infectious Disease Research and Policy. (2013) Last visited September 8, 2020. Retrieved from https://www.cidrap.umn.edu/infectious-disease-topics/pandemic-influenza
-
Claeson, M., & Waldman, R. (2019, August 09). Scientific investigation of the seventh pandemic. Retrieved from https://www.britannica.com/science/cholera/Scientific-investigation-of-the-seventh-pandemic
-
Nathanson, N., & Kew, O.M. (2010). From emergence to eradication: the epidemiology of poliomyelitis deconstructed. American Journal of Epidemiology, 172(11), 1213–1229.
-
Foster, H.D., & Hoffer, A. (2007). Hyperoxidation of the two catecholamines, dopamine and adrenaline: Implications for the etiologies and treatment of encephalitis lethargica, Parkinsons disease, multiple sclerosis, amyotrophic lateral sclerosis, and schizophrenia. Oxidative Stress and Neurodegenerative Disorders, 369-382.
-
Spreeuwenberg, P., Kroneman, M., & Paget, J. (2018). Reassessing the global mortality burden of the 1918 influenza pandemic. American Journal of Epidemiology, 187(12), 2561-2567.
-
World Health Organization. (n.d.). Report of the review committee on the functioning of the international health regulations (2005) in relation to Pandemic (H1N1) 2009 (p. 37, Rep. No. A64/10). Retrieved June 18, 2020 from https://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_10-en.pdf?ua=1.
-
Centers for Disease Control and Prevention. (2014, October 9). Marburg hemorrhagic fever (Marburg HF). Retrieved September 8, 2020: https://www.cdc.gov/vhf/marburg/resources/outbreak-table.html
-
Leblebicioglu, H., Ozaras, R., & Sunbul, M. (2017). Crimean-Congo hemorrhagic fever: A neglected infectious disease with potential nosocomial infection threat. American Journal of Infection Control, 45(7), 815-816.
-
Kofman, A., Choi, M.J., & Rollin, P.E. (2019). Lassa fever in travelers from West Africa, 1969–2016. Emerging Infectious Diseases, 25(2), 245-248.
-
UNAIDS. (2020). Global HIV & AIDS statistics - 2020 fact sheet. Retrieved from https://www.unaids.org/en/resources/fact-sheet#:~:text=GLOBAL HIV STATISTICS&text=38.0 million [31.6 million–44.5, AIDS-related illnesses in 2019.
-
Clark, M.A., Warimwe, G.M., Di Nardo, A., Lyons, N.A., & Gubbins, S. (2018). Systematic literature review of Rift Valley fever virus seroprevalence in livestock, wildlife and humans in Africa from 1968 to 2016. PLOS Neglected Tropical Diseases.
-
World Health Organization. (2015, July 24). Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Retrieved August 25, 2020, from https://www.who.int/csr/sars/country/table2004_04_21/en/
-
Centers for Disease Control and Prevention (CDC). (2009, April 30). Outbreak of swine-origin influenza A (H1N1) virus infection --- Mexico, March--April 2009. Retrieved August 25, 2020, from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0430a2.htm
-
World Health Organization. (2020, February 25). Middle east respiratory syndrome coronavirus (MERS-CoV) – The United Arab Emirates. Retrieved May 31, 2020, from https://www.who.int/csr/don/08-january-2020-mers-uae/en/
-
Centers for Disease Control and Prevention (CDC). (2019, March 08). 2014-2016 Ebola outbreak in West Africa. Retrieved June 19, 2020, from https://www.cdc.gov/vhf/ebola/history/2014-2016 outbreak/index.html#anchor_1515001464100.
-
Kindhauser, M., Allen, T., Frank, V., Santhana, R., & Dye, C. (2016). Zika: The origin and spread of a mosquito-borne virus. Bulletin of the World Health Organization, 94(9), 675-686C.
-
Worldometers. (2020, August 21). COVID-19 coronavirus pandemic. Retrieved June 18, 2020, from https://www.worldometers.info/coronavirus/
-
Bruegel, P. The Elder. (1562-1563). The Triumph of Death [Painting]. Museo Del Prado, Madrid, Spain.
-
Morens, D.M. Fauci, A.S. (2020). Emerging pandemic diseases: how we got to COVID-19. Cell. 182, 5; 1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
-
Corman, V.M., Baldwin, H.J., Tateno, A.F., Zerbinati R.M., et al. (2015). Evidence for an ancestral association of human coronavirus 229E with bats. Journal of Virology, 89(23), 11858−11870.
-
Vijgen, L., Keyaerts, E., Moës, E., Thoelen, I., Wollants, E., Lemey, P., Vandamme, A.M., & Van Ranst, M. (2005). Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. Journal of Virology, 79(3), 1595−1604.
-
Ye, Z.W., Yuan, S., Yuen, K.T., Fung, S.Y., Chan, C.P., & Jin, D.Y. (2020). Zoonotic origins of human coronaviruses 2020. International Journal of Biological Sciences, 16(10), 1686–1697.
-
Goldstein, S.A. (2017). Origins and pathogenesis of Middle East respiratory syndrome-associated coronavirus: recent advances V1. F1000Res, 6,1628.
-
Lau, S.K.P., Luk, H.K.P., Wong, A.C.P., Li, K.S.M., Zhu, L., Z., He, Z., Fung, J., Chan, T.T.Y., Fung K.S.C, & Wos, P.C.Y. (2020). Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases, 26(7).
-
McNeill, W.H. (1998). Plagues and peoples. New York: Anchor Books Publish./Doubleday, 1st edit ©1976 ISBN 9780385121224
Next story: Using NASA’s Earth Observations to Predict, Monitor, and Respond to Vector-borne and Water-related Disease
One Health Newsletter
The One Health Newsletter is a collaborative effort by a diverse group of scientists and health professionals committed to promoting One Health. This newsletter was created to lend support to the One Health Initiative and is dedicated to enhancing the integration of animal, human, and environmental health for the benefit of all by demonstrating One Health in practice.
To submit comments or future article suggestions, please contact any of the editorial board members below.