Fighting the Spread of Disease With…Words?
Authors
Justin Kastner
Department of Diagnostic Medicine/Pathobiology
Kansas State University
Megan Eppler
Graduate student
Master of Public Health program
Kansas State University
Valerie Jojola-Mount
Graduate Student
Master of Public Health program
Kansas State University
Dr. Ellyn Mulcahy
Director of Master in Public Health (MPH) program
Associate Professor, Department of Diagnostic Medicine/Pathobiology
Kansas State University
Phutsadee Sanwisate, DVM
Veterinary officer, Bureau of Livestock Standards and Certification
Department of Livestock Development
Government of Thailand;
Also presently, MPH student, Kansas State University
Kate Schoenberg
Undergraduate research assistant, Department of Diagnostic Medicine/Pathobiology
Kansas State University
Introduction
Writing from Harvard’s library in June 1926, George Sarton, one pioneer of the academic discipline of the history of science, rightly insisted that “transmission is as essential as discovery” (Sarton, 1927, p. 15). In pandemics, this is especially true as we combat large-scale disease spread. Indeed, efforts by public health practitioners to transmit scientific information are just as crucial as the original in-laboratory discoveries and epidemiological studies upon which they rely.
At a time when the planet is filled with alarm and anxiety about the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the Coronavirus Disease 2019 (COVID-19), we ought to heed these words of professor Sarton as cited above. While we need to be wary of the transmission of the novel coronavirus in our communities, perhaps we ought to be equally concerned about the transmission of information about it. They go hand in hand (if you will permit the unhygienic metaphor).
Information helps combat the spread of disease
As we (a group of faculty and students knit together through Zoom and a common interest in public health and its history) wash our hands, social distance from one another at grocery stores, and comply with state and local health department stay-at-home orders, it is easy to be obsessed with only the virus’s global and local transmission. Perhaps this is because the transmission, including community spread, of disease is familiar to us; in the few thousand years Homo sapiens have been on this planet, we have been surrounded by innumerable bacteria and viruses. Multiple authors have argued that widespread diseases, such as smallpox, plague, cholera, and now COVID-19, were results of the switch from hunter-gatherer lifestyles to agriculture (Wolfe et al., 2007). Agriculture provides more food for larger populations, but also requires a less nomadic lifestyle, and it is this combination that creates a breeding ground for disease transmission. Over the millennia, advancements in agricultural technology have enabled large groups of us to live in close proximity to each other for extended periods of time; the fact that over one-half of the U.S. (and world’s) population now lives in urban environments (like Seattle and New York) is testimony to this. Naturally (literally), the etiological agents of infectious diseases are more easily transmitted from one person to another (via cough or sneeze droplet, direct contact, and air, depending on the disease) in a way that was unseen amongst hunter-gatherer populations of early history (Ibid).
Perhaps the most vivid and fearful example of disease transmission in a population is the plague, caused by the bacterium Yersinia pestis (Y. pestis) (Figure 1). Its mere mention can give rise to chilling goosebumps. Nobody wants the plague because it sounds (and is) scary, painful, and capable of outbreaks of biblical proportions. We live in a world that has not been eradicated of this fiend; in states such as New Mexico, the plague is still a yearly visitor, though with decidedly fewer victims than the Black Death of the 14th century. While this disease can be treated with antibiotics, eradicating it remains difficult. Yet, we do not live in fear anymore that the streets of Naples, London, Marseille, or Paris will be filled with those dying of the plague, nor do we worry that all of our population will be infected should a handful of cases arise annually. Why? What has changed in seven hundred years to make this disease still an intimidating foe but a tamable one?
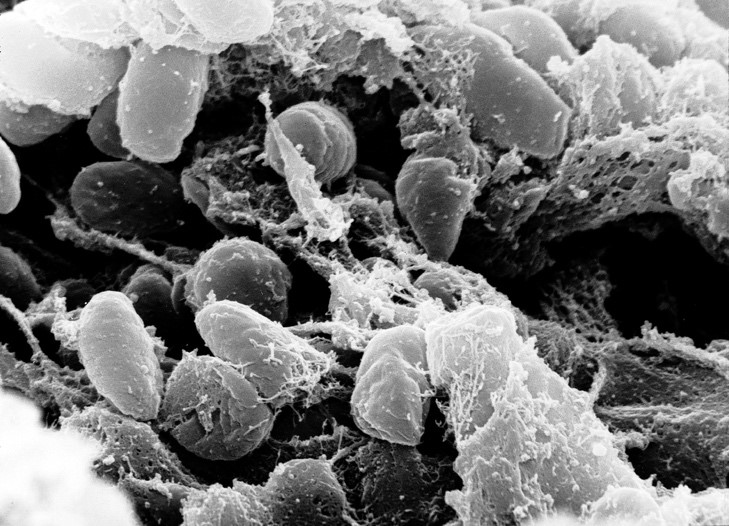
Figure 1. The agent responsible for plague, Yersinia pestis, shown under the microscope. Image from Rocky Mountain Laboratories, NIAID, NIH http://www3.niaid.nih.gov/biodefense/Public/Images.htm
In short, we now have a better understanding of how the plague is transmitted, how to treat it, and this knowledge enables us to better control its spread. Y. pestis originates in fleas that bite rodents and infect them—the first step in transmission. Rodents are long-term reservoirs for the Y. pestis bacterium, meaning that they can host the pathogen and infect others. Sometimes, these reservoir-rodents die en masse because of this infection, as was the case with the mid-1300s. Therefore, people came into contact more frequently with fleas living on rodent carcasses, in part due to increasing population density and in part due to lack of knowledge to avoid rodent carcasses. These urban victims then became infected and, many times, began to show signs and symptoms of the disease, such as buboes or pneumonia. As more people became sick and began seeking help, from either physicians or family members, they then spread the bacterium to their caregivers—further amplifying the plague’s transmission (Centers for Disease Control and Prevention, 2019, July 31).
As we have learned more about the spread of diseases, though, we have come to understand that certain pathogens, like Y. pestis, should be monitored in environments where they are known to dwell. This constitutes the first step in combatting disease transmission: surveillance. If, however, that step fails and the disease is still able to spread and infect other humans, we still have tried and true steps to curtail its spread and ability to infect. In the case of Y. pestis, we understand that once a flea has bitten a human and successfully infected the victim to the point of inducing symptoms, we can stop others from contracting plague by limiting the infected person’s contact with others—isolation. The next preventative steps, to protect caregivers from transmission, then depend upon the type of plague in question. If bubonic, doctors and nurses need to avoid direct contact with or accidental ingestion of fluids or tissues from patients. If pneumonic, they also need to avoid contact with any bacteria that individuals may exhale through the mouth or nose (Centers for Disease Control and Prevention, 2019, November 26). In practice, these are admittedly difficult measures to implement, but they have brought a marked improvement in disease control since the Black Death events of past centuries. Y. pestis, while still terrifying, is not as daunting as it once was. These improvements in disease control have come to light thanks to precisely what Harvard’s George Sarton wrote about—the transmission of scientific knowledge.
The transmission of information: a historical ally, even in ages past
|
Past pandemics, including those that occurred before the modern era, illustrate the value of information-dissemination campaigns for disease prevention. Even during the 14th century Black Death (which we sometimes, mistakenly, assume was fought without any scientific know-how whatsoever), we see attempts—albeit imperfect compared to what we know today—to study and then share (transmit to others) how to limit disease spread. Peter Ackroyd, in his history of the city of Venice, notes that in early 1348 (just as the Black Death’s full force was being exerted on Europe’s population), Venetian officials created a board “to consider diligently all possible ways to preserve the health of the city” (Ackroyd, 2009, p. 334). Ackroyd points to this board as the first recorded example of government-directed public health administration in Europe. The board must have taken their job seriously; eventually, a fourteenth-century Venetian law decreed “that Black Death corpses should be buried to a minimum depth of 5 feet” (Brown, 1998, p. 1146), which was not a bad policy in an age fraught with imperfect scientific knowledge. Without regard for their own lives (translation: with no robust personal protective equipment) (Figure 2) and with a genuine desire to serve later generations by transmitting their observation and experience with plague, physicians across Europe both treated the sick and documented in medical case-books and other so-called “plague tractates” what they were learning. In an age when the routinization of scientific observation often took a back-seat to only abstract philosophical reasoning, pioneers like Italy’s Gentile Gentili da Foligno modelled what it looked like to painstakingly chronicle the apparent causes of disease spread. Gentile, who died on June 18, 1848, while treating a sick patient, was, according to one historian, known for his then-rare “impatience with ...theorizing when practical measures [were] urgently demanded” (Campbell, 1966, p. 11).
Gentile was not the only physician interested in local disease transmission, but he and some of his likeminded colleagues’ focus on such practicalities was often overshadowed by other scholars’ preoccupation with remote causes of the outbreak. While it may seem strange to us today, during the 14th century the Black Death was attributed to a variety of distant causes (“out there” forces that, while remote, were somehow influential). These remote causes included, most spectacularly perhaps, the stars and planets. If the moon could effect changes in the tides, and if the clockwork of the night sky seemed to coordinate with other events, then (according to the logic of the day) unusual conjunctions of planets, rare eclipses, and the like could cause conditions for cataclysmic disease; both Muslim and Christian thinkers thought this, even as the courageous and practical-minded, like Gentile (mentioned above) and Ibn al-Khatib (in Spain), also defended a “theory of infection” (Sarton, 1927; Campbell, 1966, p. 28). In any case, many of their worst ideas—what we now perceive to be either outright illogical or based upon gross misinformation—were transmitted like erroneous 21st-century social media posts we see today related COVID-19 (for example, posts that mention “the use of elderberry for disease prevention,” “the COVID-19 situation is blown out of proportion,” “only old people get it,” “it won’t strike rural America,” etc.).
Dissemination of accurate and actionable information has always been problematic during pandemics. During the Bubonic Plague pandemics of yesteryear, widespread illiteracy made it difficult for written accounts or remedies to be transmitted from doctors and governments to citizens. The Venetian administration’s helpful regulations may have been easily communicated within the city of Venice, but it is unclear if or how potentially helpful information was communicated to other doctors and governments across Europe (let alone the everyday citizens who later became little more than a statistic in the estimation of plague deaths). Historian Anna Campbell, in her remarkable work The Black Death and Men of Learning, details how a number of the “big thinkers” (the “Dr. Fauci’s” of the day) cited the authoritative insights of such centers of learning as the University of Paris (a bastion, it ought to be noted, for astronomical explanations of disease phenomena) (Campbell, 1966).
In the 21st century, few people have extensive knowledge of the Bubonic Plague (or COVID-19 for that matter). The best academic sources of information about the plague are under lock and key in illustrious libraries; for this reason, as well as convenience, most curious individuals gravitate towards accessible, non-peer-reviewed, online sources regarding plague. Interestingly, many can say that they have acquired, unwittingly, a rudimentary understanding of the disease from a surprising source: a nursery rhyme! If broken down line by line, Ring Around the Rosy (Figure 3) details the general symptoms, beliefs, and occurrences during a plague outbreak. Ring Around the Rosy describes the rings that formed around the hallmark rosy-pink skin pustules on a plague victim. The jingle’s line “Pockets full of posy” reiterates the then-common belief that carrying posy (a type of flower) in one’s pocket would purify the miasmas or bad vapors in the air. “Ashes, ashes” communicates how morticians resorted to burning bodies after church cemeteries became too full. “We all fall down,” the last morbid line, paints the haunting picture of people frequently dropping dead in the street and in their homes. Ring Around the Rosy allowed children living through such dark times to identify and express their anxieties and fears about the plague in a memorable mantra. As time progressed, the rhyme was transmitted from generation to generation until the anxiety and fear behind the words waned. To this day, Ring Around the Rosy is arguably a more commonly cited source of knowledge regarding the Bubonic Plague than other information authored by scholars of that era (Siegrist, 2009).
Similar information-transmission genres are being authored today by adults, teens, and children—though in different and challenging ways. Adults in academia or those browsing the internet are bombarded by so many articles about COVID-19 that it is difficult to digest the true scope of this global pandemic. Accurate information on COVID-19 is lost as mistruths and pseudoscience are posted, liked, shared, and retweeted. Looking to the teens and children of 2020, they too are identifying and communicating fears in a similar way to the children who survived the Bubonic Plague. Instead of making catchy rhymes, their anxieties about COVID-19 are made into comedic digital media streamed through apps, memes, Tik Toks, and Snapchats. Just as Ring Around the Rosy was sung over centuries, so too will the memes of 2020 live on, transmitting our fears about COVID-19 to future generations.
Recognizing today’s efforts and yesterday’s leaders
Of course, we recognize and applaud the exemplary information-dissemination work of the U.S. Centers for Disease Control and Prevention (CDC), the European Centre for Disease Prevention and Control (ECDC), the World Health Organization (WHO), the World Organization for Animal Health (OIE), and Johns Hopkins University’s now-famous resource center (https://coronavirus.jhu.edu/). When outbreaks occur, national governments and related organizations have the principal responsibility to issue information for disease control. Because of governmental efforts to communicate, many disease outbreaks that have occurred in more recent history—such as polio, measles, rubella, and diphtheria—have been controlled effectively by vaccination. The collaboration of national governments and international scientific organizations plays an especially essential role in global health emergencies. The WHO’s coordinated international outbreak response using resources from the Global Outbreak Alert and Response Network (GORAN) helped control Severe Acute Respiratory Syndrome (SARS) in 2002-2003. This network combined experts and technical resources for rapid identification, confirmation, and response to outbreaks of international importance (Institute of Medicine et al., 2004). One can imagine how such networks could have assisted the Gentile da Foligno’s and the Ibn al-Khatib’s of ages past! The transmission of information among neighboring countries worldwide is necessary to control outbreaks across international borders. For instance, in Southeast Asia, the Mekong Basin Disease Surveillance (MBDS) network consists of Cambodia, China, Lao PDR, Myanmar, Thailand, and Vietnam; these countries’ governments collaboratively share epidemiological intelligence across their shared borders. The relative success of such regional disease surveillance networks can mitigate the threat of infectious disease outbreaks (Bond et al., 2013).
Transmission of disease-related information to people who have been directly or indirectly affected during disease outbreaks is crucial. According to A.J. Tumpey and others (2018), individuals’ risk perception differ depending on “how likely they think the actual hazard will affect them personally and their beliefs about how severe the harm might be.” When an outbreak event causes high concern or anxiety, the level of risk perception will be high. In such cases, it is especially critical to have credible voices sharing the best information. Therefore, governments should be scientifically competent but also work to gain trust and earn credibility. During an outbreak where risk perception varies amongst the masses, trust and credibility are needed to induce people to follow rules (e.g. stay-at-home orders) and, indeed, comply with all of the public health authorities’ recommendations. The ability to successfully combat disease outbreaks hinges on effective information sharing and coordination among governments, public health organizations, non-governmental organizations, and other civic and scientific groups.
The dissemination of information regarding disease does not happen in cold, institutional fashion; real people—physicians, microbiologists, epidemiologists, communications directors, and veterinarians—are involved. One noteworthy person who worked to improve public health in his day was Germany’s Robert Koch, whose career benefited from prior microbiology pioneering including, but not limited to, Louis Pasteur. He famously and uniquely demonstrated the existence of the germ theory in 1882 through his discovery of Mycobacterium tuberculosis that causes tuberculosis (Centers, 1982). In part due to Koch’s discoveries, we have rightly looked to science to try and learn more about disease control. Prior to Koch, a good number of doctors and scientists turned to treatments we now know would have accomplished very little (bloodletting is a good example). Indeed, the transmission of the germ theory of disease allowed for a drastic improvement in public health practices. Scientific pioneers like Koch were convinced that the “germ theory” was true, and this landmark microbiological discovery certainly earned the respect and captured the attention of other European scientists—among them the budding writer and physician, Dr. Arthur Conan Doyle. Despite the press Koch received and an emerging scientific consensus amongst the world’s microbiological elite, the germ theory, and other now-taken-for-granted allied principles, were only slowly accepted by medical and veterinary scientists during the last quarter of the 19th century. This posed a problem during Koch’s era, as many public health threats were (like COVID-19) of animal origin. Combatting such zoonotic diseases required a new kind of harmony of scientific understanding between the medical and veterinary professions. Public health depended on such a harmony, which came into tune over a century ago in a neighborhood now, in 2020, embattled with COVID-19.
|
In Brooklyn, New York in 1878, a landmark moment for public health occurred when, for the first time in America, a veterinarian was appointed to a local, municipal board of health. Dr. Lachlan McLean—who studied veterinary medicine in Edinburgh, Scotland, and enjoyed a short career not far from Loch Ness—had emigrated to the U.S. in 1875 (Figure 4). Appointed Veterinary Medical Inspector for the Brooklyn Board of Health three years later, he worked aggressively to improve public health conditions in Brooklyn. No doubt because of his convictions about disease transmission, he quickly became a whistleblower of sorts, exposing poor animal health conditions all throughout the city. From the contamination of milk, to unsanitary practices surrounding the feeding of garbage to beef and dairy cattle, to an equine disease outbreak, he covered it all. A kind of Gentile da Foligno of his day, Dr. McLean was respected by some but resented by others. He made enemies, both on dairy farms and in Brooklyn’s health department, as he actively sought to keep the public safe.
While he made important contributions to milk safety, he was also involved in exposing an outbreak of pleuro-pneumonia in cattle. Pleuro-pneumonia is a respiratory disease not transmissible to humans, but during the last quarter of the 1800s, it was of major economic importance as American cattle exported to Britain were suspected of spreading it. As diplomats argued over who was spreading this disease and how best to contain it, Dr. McLean participated in a kind of covert scientific investigation—first looking into a suspect herd of cattle on the edge of Brooklyn. After examining them, he diagnosed the herd as harboring the disease. His diagnosis was disputed by others (especially those in the distillery and dairy industry), and not finally confirmed until more post-mortem examinations were completed. Once he was vindicated in his diagnosis, however, he was authorized by the local government to take charge of the situation and through his leadership, he “effectively stamped out the disease” (Hughes, 1913, p. 206).
Dr. McLean was able to effect real change not only in the Brooklyn area but across the country as his career in America unfolded. He worked tirelessly as an exemplary leader in public health to transmit information that would help both veterinarians and physicians stop outbreaks in their tracks. He often argued with his own colleagues and even risked his professional reputation because of his convictions about and commitment to public health. The late and distinguished Dr. Jim Steele, in an article devoted to the history of veterinary public health in America, describes McLean’s future-minded insights: “McLean considered that the time was fast approaching when society at large would expect and demand more of the veterinary profession in the way of certifying the healthy condition of [foods of animal origin]” (Steele, 1991, p. 958). McLean’s commitment, personal sacrifice, and vision made him an influential veterinarian in the field of public health. When local health departments look for courage to transmit vital public health information, they can look to Dr. McLean’s example.
Information-transmission lessons from HPAI and SARS
Harvard’s George Sarton told us that “transmission is as essential as discovery.” A more recent soothsayer, Max Brooks, a teller of tales such as World War Z, that now appear to be coming to life in macabre theater on the world stage, tells us that our response to this new virus has been lethargic and lacks organization (Gross, 2020). Has our response been hindered by a lack of timely communication and a lack of data? Has the transmission of information been interrupted or slowed down, much like how we would want this virus to be slowed? If so, how do we decrease transmission of disease whilst increasing transmission of information? We have countless boards of health and agencies—such as that first one in 1348 in Venice. How then can we clearly and succinctly transmit information? In a crisis such as this pandemic, communication should follow broadly accepted international principles of communication that are effective worldwide: (1) be honest and transparent in order to build credibility; (2) be consistent, use evidence and data to clearly explain what is happening; (3) communicate often as people in times of crisis will be worried and anxious, and look for regular and situational updates; (4) be empathetic and aware of stress and anxiety during the crisis; and (5) remember that this is all part of governance. The truth of these principles is confirmed by recent outbreak communication efforts.
In 2004, an outbreak of Highly Pathogenic Avian Influenza (HPAI) occurred in Thailand resulting in some exceptional numbers of human illness and death. Thailand’s Ministry of Public Health (MOPH) conducted public health education and risk communication campaigns to educate people, especially those in the high-risk group (i.e., poultry farmers and rural residents), about AI infection and “self-help” disease prevention. The messages from the campaign were transmitted to people via public media, local health personnel, and health volunteers. This campaign was, in a word, successful. People followed the recommendations from MOPH, implementing effective poultry disease outbreak prevention and control, and this resulted in no reported human illnesses or deaths from HPAI in Thailand in 2007.
The transmission of information was critical to national and global governance in the 2003 SARS outbreak. In a risk-communication review conducted one year after this global event, David Byrne (then European Union’s Commissioner for Health and Consumer Protection) credited the European Commission’s efforts to transparently pass along, daily reports of SARS cases in Europe. When the number of new SARS cases eventually began to drop (something we all presently long for with COVID-19), it was logical for the media and the public to trust their governments that the outbreak was truly sun-setting. Commissioner Byrne rightfully concluded that such transparency enhanced EU governance—in stark contrast to the bovine spongiform encephalopathy (BSE, or mad cow disease) crisis of the late 1990s and early 2000s, when a number of European governments gave out incorrect information that they had to later necessarily contradict (Duncan, 2004).
The One Health tradition, the COVID-19 response, and today’s distinct advantage
This summer (June 25) marks the 13th anniversary of the American Veterinary Medical Association’s “One Health” resolution (Kahn et al., 2008). Since this 2007 declaration, much progress has been made by physicians, veterinarians, and public health practitioners in the common fight against disease, especially zoonotic threats. This article, however, sought to lengthen our perspective—and our appreciation—for the centuries-long efforts to collaborate and communicate disease-control best practices. Both distant and recent pasts reveal that collaborative communication during a disaster response is vital for efficient and accurate transmission of information. An important part of this process is to build trust by establishing transparent methods of communicating with the public (Savoia, Lin, and Gamhewage, 2017). We are all witnessing the importance of trust and transparency in the current COVID-19 crisis: state health departments have rolled out 24/7 staffed telephone lines to answer questions, and local health departments have deployed electronic screening processes for people to have their initial symptoms screened and are publicizing those results on a weekly or even daily basis. In addition, local school districts are playing a much-needed role in food distribution. All of these state and local government efforts build trust and credibility amongst their constituents. Amongst all these efforts, and those of the CDC and the WHO, we celebrate the transmission of information. Local, state, federal, and global health communicators in 2020 have more methods for transmitting information than ever before. Gentile da Foligno and Ibn al-Khatib would no doubt be impressed. A polite nod of confidence from Robert Koch can be imagined. We can see Lachlan McLean smiling in approval.
And Professor Sarton would, surely, be most pleased.
References
Ackroyd, P. (2009). Venice: Pure City. New York: Anchor Books.
Bond, K.C., Macfarlane, S.B., Burke, C., Ungchusak, K., & Wibulpolprasert, S. (2013). The evolution and expansion of regional disease surveillance networks and their role in mitigating the threat of infectious disease outbreaks. Emerging Health Threats Journal, 6.
Brown, P. (1998). BSE: The final resting place. Lancet, 351(9110), 1146-1147.
Campbell, A.M. (1966). The black death and men of learning. New York: AMS Press.
Centers for Disease Control. (1982). Historical perspectives centennial: Koch’s discovery of the tubercle bacillus. MMWR Morb Mortal Wkly Rep. 31(10), 121-123. https://www.cdc.gov/mmwr/preview/mmwrhtml/00000222.htm
Centers for Disease Control and Prevention. (2019, July 31). Ecology and Transmission. https://www.cdc.gov/plague/transmission/index.html
Centers for Disease Control and Prevention. (2019). Frequently asked questions. https://www.cdc.gov/plague/faq/index.html?CDC_AA_refVal=https://emergency.cdc.gov/agent/00_modules/plague/faq.asp.
Chunsuttiwat, S. (2008). Response to avian influenza and preparedness for pandemic influenza: Thailand’s experience. Respirology, 13(s1), S36-S40.
Duncan, B. (2004). Public Risk-Perception and Successful Risk-Communication. Institute for Prospective Technological Studies Report, 0(82), 26-30.
Goetz, T. (2014). The Remedy: Robert Koch, Arthur Conan Doyle, and the Quest to Cure Tuberculosis. New York: Gotham Books and the Penguin Group.
Gross, T. (2020). 'All of this panic could have been prevented': Author Max Brooks on COVID-19. https://www.npr.org/2020/03/24/820601571/all-of-this-panic-could-have-been-prevented-author-max-brooks-on-covid-19
Hughes, D.A. (1913). Members of the Royal College of Veterinary Surgeons who have been makers of American Veterinary History: A postscript. British Veterinary Journal, 69, 202-208.
Institute of Medicine (US) Forum on Microbial Threats; Knobler, S., Mahmoud, A., Lemon, S., Mack, A., Sivitz, L., & Oberholtzer, K., editors (2004). The public health response to SARS. In Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK92460/
Kahn, L.H., Kaplan, B., Monath, T.P., & Steele, J.H. Teaching "One Medicine, One Health". (2008). American Journal of Medicine, 121(3), 169-170.
Sarton, G. (1927). Introduction to the History of Science, Volume 1. Baltimore: The Williams and Wilkins Company.
Savoia, E., Lin, L., & Gamhewage, G.M. (2017). A conceptual framework for the evaluation of emergency risk communications. American Journal of Public Health, 107(S2), S208-S214.
Siegrist, M. (2009). Bubonic plague: History and epidemiology. International Journal of Global Health and Health Disparities, 6(1), 132-142.
Steele, J.H. (1991). History of veterinary public health in the United States of America. Revue scientifique et technique (International Office of Epizootics), 10(4), 951-983.
Next Story: COVID-19 pandemic, present and future: What strategies should we take?
One Health Newsletter
The One Health Newsletter is a collaborative effort by a diverse group of scientists and health professionals committed to promoting One Health. This newsletter was created to lend support to the One Health Initiative and is dedicated to enhancing the integration of animal, human, and environmental health for the benefit of all by demonstrating One Health in practice.
To submit comments or future article suggestions, please contact any of the editorial board members below.