Wildlife and One Health: From Jackal to Humans
Within the framework of One Health, the role of biodiversity and ecology of parasites in wildlife has not received adequate attention (Viljoen 2017). The current focus on surveillance and control strategies of infectious diseases does not follow a holistic approach. For example, some studies that investigated the epidemiology of infectious diseases barely examined the ecosystem as whole. The interrelationships between humans, animals, and the parasite microbiome are not fully understood. One Health has a pivotal role to play, because of its fundamental principles that are inclusive of all aspects that can contribute to disease monitoring and control. One Health is defined as a collaborative, multisectoral, and transdisciplinary approach to achieving optimal health of ecosystems, considering the interconnection between animals, plants, humans, and their environment (CDC 2018). It considers that animals exist with humans in a diverse ecosystem that has varied environmental factors, such as nutrition, toxins, climate change, and infectious agents (Deem et al. 2001). A key aspect of One Health is fieldwork because it determines the extent of mitigation in terms of methods employed to control diseases and ensure optimal ecosystem health. It also identifies gaps and barriers that would derail the monitoring and control processes.
A One Health Model of Vector Borne Zoonotic Disease
Environmental changes have influenced tick-borne pathogen distribution, disease epidemiology, and new host establishment. For example, in the United States, Babesia microti has been identified in areas where Borrellia garinni is prevalent, noted as two parasites transmitted by the same tick vector Ixodes scapularis ( I. scapularis) (Vannier et al. 2015). New host establishment should be anticipated when dealing with infections originating in an environment that includes multiple possible hosts. Due to the occurrence of multiple pathogens in the environment, cases of misdiagnosis and mistreatment will be eminent, such as the case of babesiosis and Lyme disease in patients in the United States (Citera et al. 2017). The above mentioned case should be informed through the fieldwork approach of One Health, to consider all factors present within an ecosystem including the parasite microbiome, potential hosts, and their historical and evolutionary relations to disease. Most endemic hosts of parasites are wildlife hosts, because of their long standing relationship with the parasite in the specific habitat, as was the case with B. rossi and indigenous wild canids such as black-backed jackals and African wild dogs (Penzhorn et al. 2017).
Therefore, the reported study addressed the role of wildlife as endemic hosts in the lifecycle of parasites, such as Babesia, and the control of babesiosis. An understanding of the historical and evolutionary perspective of the parasite can help inform possible mitigation strategies related to disease transmission in the ecosystem. The infection status is informed by surveillance using clinical evidence based on overt signs of infection, genomics and proteomics of the parasite, and vector characterization. Through this approach of the role of wildlife in the natural transmission of a pathogen, subsequently a model was proposed to elucidate the zoonotic complex cycle of parasites from wildlife, domestic animals, and humans. The model provide baseline of information about the parasites interrelations with hosts, and makes speculations on future establishments in new potential hosts.
A Compulsory Fieldwork to Unveil a Complex Parasitic Cycle
The focus is on the occurrence of Babesia rossi (B. rossi) in black-backed jackals, African wild dogs, and domestic dogs living in South Africa. Based on phylogenetic analysis of the B. rossi 18S rRNA gene, there are similarities between the B. rossi that occurs in domestic dogs and indigenous wild canids. These findings prompted the use of a fieldwork model for One Health that places wildlife as the starting point of inquiry when it comes to infectious diseases. This is due to the role most wild species play in the ecosystem as natural reservoirs hosts of pathogens and their impact on infection to susceptible hosts such as humans and domestic animals. Following this model, data was collected to establish possible fieldwork methods based on a One Health approach. The understanding of Babesia transmission is targeting B. rossi infections in susceptible domestic dogs and wild canids such as black-backed jackals which can inform researchers when developing studies on other zoonotic pathogens such as B. microti. The findings of my research informed the starting point of inquiry, focusing on the relationship of the B. rossi isolates from its natural reservoir host, the endemic species black-backed jackal, to the most susceptible host, domestic dogs. This finding would provide information about the evolution of the parasite through its interaction within hosts, as the parasite-host relationship is an indicator of the best fieldwork management practice of diseases. For example, domestic dog hosts act as sentinels to multiple parasites including zoonotic parasites such as Lyssavirus which is responsible for rabies. Rabies infections were reported to cause threats in African wild dog populations in Madikwe Game Reserve, South Africa (Sabeta et al. 2018). Phylogenetic analysis of the partial nucleoprotein gene sequence indicated a link between wildlife and domestic dogs rabies cycles in South Africa (Sabeta et al. 2018). The same isolate transmission to human could be anticipated. Control measures rely on the knowledge of virus genomics and proteomics for development of vaccine which can manage the infection in all affected populations including, wildlife, domestic dogs, and humans. The Babesia management practices based on a fieldwork approach can be adopted to control zoonotic Lyssavirus and other parasites such as B. microti.
The Canine Babesiosis Case
Babesia is an intraerythrocytic protozoan parasite of phylum Apicomplexan and family Babesidae (Neitz 1947). It was discovered by Victor Babes (1888) in Romanian cattle, and later the species was named Babesia bovis. The life cycle of B. rossi starts in the tick vector, following transtidal and transovarial transmission modes, and further completes its metamorphosis in vertebrate hosts such as domestic animals, rodents, humans, and wildlife (Figure 1). Three species of Babesia which infect domestic dogs and wild canids namely: B. rossi, B. vogeli, and B. canis. Species differ on the basis of pathogenicity and vector specificity (Carret et al. 1999). The South African strain of Babesia in canids is called B. rossi, and its initial discovery was from an indigenous wild canid host, the side-backed jackal in Kenya (Nuttall 1910). It is transmitted by a tick vector Haemaphysalis reticulatus (Apanaskevich et al. 2007), which has a wide distribution in the southern parts of Africa, except in Namibia where arid conditions favor Rhipicephalus sanguines. B. rossi is the most virulent of all the strains of Babesias in dogs, with varied clinical presentation in susceptible domestic dogs. Clinical presentation is associated with different genotypes of an antigen gene called B. rossi erythrocytes membrane antigen 1 (BrEMA1) (Matjila et al. 2009). It was discovered that this gene is absent in other isolates within the African continent. Molecular characterization of B. rossi using real-time PCR assay specific for B. rossi (BrEMA1) revealed the absence of BrEMA1 in Nigerian isolates, this was postulated as a contributing factor for domestic dogs to cope better with infection (Adamu et al. 2014).
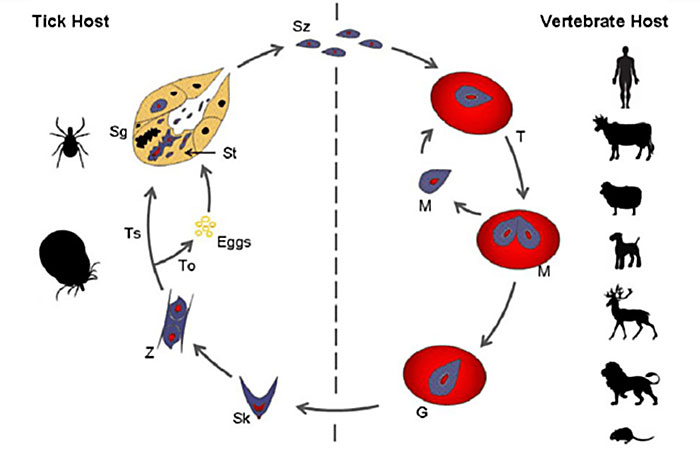
Figure 1. General Schematic Representation of the Babesia Life Cycle in Vector and Hosts. Babesia sporozoites are represented by (Sz), which are injected into the bloodstream of a vertebrate host with saliva, during the blood meal of an infected tick. After invading the erythrocytes they differentiate into trophozoites (T), which undergo merogony (M) forming two or four merozoites. (Adapted from Mehlhorn & Schein 1985)
Understanding the life cycle of parasites is fundamental to the fieldwork approach because it provides an understanding of the biology as the parasite undergoes phases of transmission. It can inform researchers about appropriate fieldwork methods for management practices such as surveillance and disease control. The initial method of identifying B. rossi is based on clinical presentation of susceptible hosts, often dogs that show signs of anemia, hepatopathy, and multiple organ dysfunction in extreme cases (Schetters et al. 2009). Follow up methods such as light microscopy are used to differentiate the appropriate parasite based on the size and morphological appearance of the intra-erythrocytic forms of the parasite in peripheral blood smear (Levine 1988; Van Heerden et al. 1983). This is a simple and inexpensive method of detection, but has low sensitivity when parasitaemia is low in atypical and chronic cases. Morphological identification paired with historical knowledge of tick vectors that occur in specific areas also guide the fieldwork approach to diagnosis. Molecular diagnostic tools are also used to validate diagnosis because they are considered more reliable and are widely used for the detection/characterization of parasitic blood infections in hosts and tick vectors (Birkenheuer et al. 2004). Advances in molecular methods, such as Polymerase Chain Reaction (PCR) and Reverse Line Blot (RLB) hybridization assays, are invaluable in detecting multiple infections within pathogens of various species as well as identification of subspecies or species variants (Nagore et al. 2004).
Fieldwork: Methods and Strategies
Diagnostic techniques mentioned above allow pathogen detection in wild animals or reservoir hosts, where both mixed species and mixed genus infections may be present (Penzhorn et al. 2017). Species specific differential diagnostic methods such as SYBR green and Taqman probe real-time PCR assays for B. rossi were valuable to discriminate between different genotypes of the same parasitic isolates (Matjila et al. 2009; Troskie et al. 2019). Automated sequencing and phylogenetic sequence analysis were breakthroughs in providing an understanding of the evolutionary relationships of species from genes. Examples of fieldwork application in wildlife diagnosis are numerous. A recent study revealed occurrence levels of B. rossi in domestic dogs, African wild dogs and black-backed jackals (Shabangu, 2018). The RLB hybridization assay results showed B. rossi was present in 66 domestic dogs (88%) at the outpatient clinic in Ondesterpoort veterinary academic hospital, 5 (10%) free-ranging African wild dogs from Kruger National Park, 22 (29%) free-ranging black-backed jackals from the Mogales Gate Biodiversity Center, and 7 (28%) captive black-backed jackals from S.A Lombard Nature Reserve. Phylogenetic analysis of the near full-length 18S rRNA gene sequence confirmed the occurrence of B. rossi in black-backed jackals and domestic dogs. These methods were able to indicate that wild canids and domestic dogs share similar tick-borne parasites highlighting the importance of field work in tick control especially for more susceptible domestic dogs.
This fieldwork approach for diagnosis of B. rossi in canids is valuable to One Health (Figure 2). Methods similar to RLB hybridization assay and Taqman probe real-time PCR assay can be used to unravel co-infections caused by zoonotic infections, address hidden pathology, and the interaction of diseases in humans to cases such as B. microti and B. garinni co-infectionsin patients from the USA. Research should also focus on all aspects of the lifecycle zoonotic parasites such as B. microti, specifically on its modes of transmission from its tick vector I. scapularis to its endemic host (e.g. white-tailed deer) or susceptible human hosts. Understanding the biology of zoonotic parasites can inform the control of human babesiosis. The parasite-host relationship can be understood through the proposed fieldwork method. In the case of South Africa where malaria is prevalent (i.e., Kruger National Park), the use of differential diagnostics is important in determining the actual prevalence of malaria or possible babesiosis infections which share similar clinical symptoms in humans.
|
Figure 3. Domestic Dogs and Human. Dogs and humans are the highly susceptible hosts of Babesia rossi as a One Health model. (Credit: Dr. Francis Kolo, Mnisi Village, at Kruger National Park in South Africa) |
One Health as a Guide of Public Health Fieldwork
Despite the mentioned success, the proposed model of fieldwork should consider all elements that contribute to disease control. There is minimal collaboration in this model of surveillance, as it often isolates the human community in the ecosystem and the role they play in disease monitoring and control. The important role of One Health in fieldwork is to establish scientific collaborations and partnerships when related to disease surveillance through consultations with communities at risk. An example of an effective approach to surveillance is the community-based One Health program in the Mnisi Community, outside the Kruger National Park, in South Africa which is a collaboration between the University of Pretoria, Peace Parks, and the community of Mnisi village outside the Kruger National Park (Figure 3). Within the collaboration a network of local and international researchers in various research fields, clinicians in the veterinary and medical field, conservationists, wildlife managers and rangers are all working together to ensure ecosystem health. Additionally, the program includes education on risk assessment and risk mitigation. The recent pilot tests involved over 98% (n = 82) of community members namely, traditional leaders, health care workers, teachers, and pastoralists (Berrian et al. 2018). Research is undertaken in collaboration with community members, and scientific research findings are communicated back to the community to help improve on management practices. Hence, the role of wildlife in the ecosystem is not receiving adequate attention when it comes to disease control within this program. There was also no emphasis placed on the role of wildlife despite the community living in close proximity to conservation areas, leaving the community at increased risk of infection.
One Health serves as a holistic approach to guide researchers and practitioners in their initiatives to ensure effective risk assessment and disease control. The historical aspects of the community, including culture and tradition, can inform scientific inquiry. It is important to establish formal relationships with conservationists, wildlife managers, farmers, public health experts, researchers, community members, and academic institutions to enhance awareness and strengthen clinical/scientific fieldwork approaches in One Health.
References
Adamu, M., Troskie, M., Oshadu, D.O., Malatji, D.P., Penzhorn, B.L. & Matjila, P.T. (2014). Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau State, Nigeria. Parasit Vectors, 7 (1), 119.
Apanaskevich, D.A., Horak, I.G., & Camicas, J.L. (2007. Redescription of Haemaphysalis (Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma) leachi group from East and southern Africa, and of Haemaphysalis (Rhipistoma) leachi (Audouin, 1826) (Ixodida, Ixodidae). Onderstepoort J Vet Res, 74 (3), 181-208
Babes, V. (1888). Sur l'hemoglobinurie bacterienne du boeuf. Comptes Rendus de l'Academie de Sciences, 692-694.
Berrian, A.M., Smith, M.H., van Rooyen, J., Martínez-López, B., Plank, M.N., Smith, W.A., & Conrad, P.A. (2018). A community-based One Health education program for disease risk mitigation at the human-animal interface. One Health, 5, 9-20.
Birkenheuer, A. J., Neel, J., Ruslander, D., Levy, M., & Breitschwerdt, E. (2004). Detection and molecular characterization of a novel large Babesia species in a dog. Vet. Parasitol, 124, 151-160.
Carret, C., Walas, F., Carcy, B., Grande, N., Précigout, E., Moubri, K., Schetters, T. P., & Gorenflot, A. (1999). Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. J Eukaryot Microbiol. 46, 298-301.
Center for Disease Control and Prevention. (2018). One Health Basics. Retrieved from https://www.cdc.gov/onehealth/basics/index.html.
Citera, M., Freeman, P.R., & Horowitz, R.I. (2017). Empirical validation of the Horowitz multiple systemic infectious disease syndrome questionnaire for suspected Lyme disease. Int J Gen Med, 10, 249.
Deem, S.L., Karesh, W.B. & Weisman, W. (2001). Putting Theory into Practice: Wildlife Health in Conservation. Conserv. Biol, 15, 1224-1233.
Levine N.D. (1988). The Protozoa Phylum Apicomplexa CRC Press, Boca Raton, Florida
Matjila, P. T., Carcy, B., Leisewitz, A.L., Schetters, T, Jongejan, F, Gorenflot, A., & Penzhorn, B.L. (2009). Preliminary evaluation of the BrEMA1 gene as a tool for associating Babesia rossi genotypes and clinical manifestation of canine babesiosis. J Clin Microbiol, 47, 3586-3592.
Mehlhorn, H., & Schein, E. (1985). The piroplasms: life cycle and sexual stages. Adv. Parasitol, 23, 37-103.
Nagore, D., Garcıa-Sanmartın, J., Garcıa-Pérez, A.L., Juste, R.A. & Hurtado, A. (2004). Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Northern Spain. Int J Parasitol, 34, 1059-1067.
Neitz, W. & Steyn, H. (1947). The transmission of Babesia canis (Piana and Galli-Valerio, 1895) to the black-backed jackal [Thos mesomelas (Schreber)], with a discussion of the classification of the piroplasms of the Canidae. J S Afr Vet Assoc, 18, 1-12.
Nutall, G. (1910). On hematozoa occuring in wild animals in Africa. Parasitiology, 3, 108-116.
Penzhorn, B.L., Vorster, I., Harrison-White, R.F., & Oosthuizen, M.C. (2017). Black-backed jackals (Canis mesomelas) are natural hosts of Babesia rossi, the virulent causative agent of canine babesiosis in sub-Saharan Africa. Parasit Vectors, 10, 124.
Sabeta, C.T., Rensburg, D.D., Phahladira, B., Mohale, D., Harrison-White, R.F., Esterhuyzen, C., & Williams, J.H. (2018). Rabies of canid biotype in wild dog (Lycaon pictus) and spotted hyaena (Crocuta crocuta) in Madikwe Game Reserve, South Africa in 2014-2015: Diagnosis, possible origins and implications for control. JSA Vet Assoc, 89(1), 1-13.
Schetters, T.P., Kleuskens, J.A., Van De Crommert, J., De Leeuw, P.W., Finizio, A.L. & Gorenflot, A. (2009). Systemic inflammatory responses in dogs experimentally infected with Babesia canis; a haematological study. Vet. Parasitol, 162, 7-15
Shabangu, N. (2018). Occurrence of Babesia rossi in domestic dogs, black-backed jackals and African wild dogs in South Africa (Master’s dissertation, University of Pretoria).
Troskie, M., De Villiers, L., Leisewitz, A., Oosthuizen, M.C., & Quan, M. (2019). Development and validation of a multiplex, real-time PCR assay for Babesia rossi and Babesia vogeli. Ticks Tick-borne Dis, 10(2), 421-432.
Van Heerden, J., Reyers, F., Stewart, C.G. (1983). Treatment and thrombocyte levels in experimentally induced Canine ehrlichiosis and Canine babesiosis Onderstepoort. J. Vet. Res, 50, 267-270
Vannier, E.G., Diuk-Wasser, M.A., Mamoun, C.B., & Krause, P.J. (2015). Babesiosis. Infect Dis Clin, 29, 357-370.
Viljoen, S. (2017). Wildlife health in human-modified landscapes: epidemiology of tick-borne pathogens affecting black-backed jackals and caracals (Doctoral dissertation, University of Cape Town).
Author
University of Pretoria, Department of Veterinary and Tropical Diseases (previous),
Department of Education (current), Hatfield x 0110, Pretoria, South Africa
Next Story: The Pasteur Institute and Global Health
One Health Newsletter
The One Health Newsletter is a collaborative effort by a diverse group of scientists and health professionals committed to promoting One Health. This newsletter was created to lend support to the One Health Initiative and is dedicated to enhancing the integration of animal, human, and environmental health for the benefit of all by demonstrating One Health in practice.
To submit comments or future article suggestions, please contact any of the editorial board members.

